Start Your Journey
Start Your Pharmacy the Right Way
Complete guidance to build your compliant pharma business in India
INTRODUCTION
India’s pharmaceutical industry is a global powerhouse, ranked 3rd worldwide in production volume. A significant part of this industry is the vast network of retail and wholesale pharmacies – more than 850,000 retail pharmacy outlets spread across urban and rural areas. Starting a pharmacy business (whether a neighbourhood medical store or a wholesale distribution venture) involves more than just stocking medicines; it requires meticulous compliance with Indian laws and regulations. Entrepreneurs must navigate business incorporation, obtain multiple approvals, secure mandatory licenses and ensure qualified personnel are in place. This comprehensive guide explains everything required to start an allopathic pharma business (retail or wholesale) in India – from choosing the right business structure and obtaining necessary approvals/licenses, to understanding why these compliances are needed. We will also address frequently asked questions to help you kick-start a pharmacy venture with full legal compliance and confidence.
TYPE OF BUSINESS SUITABLE FOR A PHARMA INDUSTRY
Choosing the right business structure is a crucial first step in establishing a pharma retail or wholesale business. In India, you can operate a pharmacy under various legal entities – sole proprietorship, partnership firm, Limited Liability Partnership (LLP) or a private limited company. Each structure has its pros and cons:
- Sole Proprietorship: A simple, single-owner setup ideal for small pharmacies. It allows fast decisions but offers no liability protection and owner is personally responsible for all debts and legal risks.
- Partnership Firm: Suitable for joint pharmacy ventures, especially among family or friends. Responsibilities and profits are shared, but partners face unlimited liability. Registration is optional but advisable for legal clarity.
- Limited Liability Partnership (LLP): LLPs combine partnership flexibility with limited liability protection. They're easier to manage than companies and suitable for pharmacies seeking legal separation and lower compliance without compromising ownership control.
- Private Limited Company: Ideal for large or multi-branch pharmacy businesses. Offers limited liability, better funding options and brand credibility, though requires strict compliance with audits, filings and statutory reporting.
Which structure is best? It depends on the scale and vision of your pharma venture. For a single retail chemist shop, many opt for a sole proprietorship or simple partnership for ease of operation. If you seek limited liability and future expansion, an LLP or private limited company is best suited. For example, converting to a company can make it easier to get investments or franchises. It’s important to choose a structure at the outset that aligns with your business goals – you must register the pharmacy under one of these forms before applying for licenses. Consulting a legal expert can help in deciding the optimal structure, but ensure that the business entity (whether proprietorship, firm, LLP or company) is duly registered before moving on to the next steps.
NECESSARY APPROVALS AND REGISTRATIONS
- Business Incorporation/Registration: Register your pharmacy under the chosen business structure—proprietorship, partnership, LLP or company. This gives legal identity and is required for licenses like GST, drug license and tax compliance.
- Trade License from Local Authority: A municipal trade license allows legal operation from the chosen premises. It ensures local body approval for your pharmacy and compliance with sanitation, safety and operational guidelines.
- Shops and Establishment Act Registration: Mandatory for all businesses, this registration governs employment terms, working hours and holidays. Apply within 30 days of opening your pharmacy to legally employ staff and meet labour regulations.
- Tax Registrations (PAN, TAN and GST): PAN, TAN and GST registrations are essential for tax compliance. GST is required if turnover exceeds ₹40 lakh or for inter-state sales. These enable smooth procurement and legal billing.
- Professional Licenses for Staff – Pharmacist Registration: A qualified, State Pharmacy Council–registered pharmacist is mandatory for retail pharmacies. Their appointment is crucial for obtaining a drug license and ensuring proper dispensing of medicines.
- Premises Verification and Infrastructure: Drug license approval requires a compliant shop with 10–15 sq. meters space, proper construction and cold storage for sensitive medicines. A floor plan and photos may be required.
- Fire Safety NOC: Pharmacies storing chemicals or drugs may need a Fire NOC. It ensures the premises meet fire safety standards like extinguishers, emergency exits and alarms to protect life and inventory.
- Environmental/Pollution Control Approval: Pharmacies generating expired or biomedical waste may require Pollution NOC. Especially for wholesalers, this ensures proper disposal of pharmaceutical waste via authorized handlers and meets environmental norms.
Each of the above approvals should be obtained in the appropriate sequence. Typically, you will incorporate the business first, then get tax registrations, then apply for local trade/shop licenses, while concurrently preparing for the drug license application. In the next section, we discuss the core licenses required under drug control laws.
REQUIRED LICENSES FOR A PHARMACY BUSINESS
The green cross-in-circle is the official identification mark of licensed pharmacists in India, often displayed by medical stores to signify the presence of a registered pharmacist.
Once your business is registered and preliminary approvals are in place, you must obtain specific licenses under the Drugs and Cosmetics Act, 1940 to legally sell or distribute medicines. Operating without these licenses is illegal and punishable under Indian law. The exact type of drug license you need depends on the nature of your pharma business – retail pharmacy (chemist shop) or wholesale distribution (stockist). Below are the essential licenses required:
- Retail Drug License (Chemist Shop License): A Retail Drug License is mandatory for anyone operating a pharmacy or chemist shop selling allopathic medicines to the public. It is issued by the State Drug Control Authority for a specific location. To obtain this license, you must appoint a registered pharmacist who holds a valid Pharmacy Registration Certificate from the State Pharmacy Council. The premises must be at least 10 square meters, clean, ventilated and equipped with cold storage for temperature-sensitive drugs. Key documents required include the pharmacist’s qualification proof, appointment letter, business incorporation papers, property/rental documents, premises layout, an affidavit of compliance with the Drugs and Cosmetics Act and the prescribed government fee challan. Upon approval, the license is issued in Form 20 (for general drugs) and Form 21 (for restricted drugs). The license must be clearly displayed at the shop and is valid for 5 years, after which it must be renewed. This license legally allows you to stock, dispense and sell medicines to customers against valid prescriptions.
- Wholesale Drug License: A Wholesale Drug License is required for businesses engaged in the wholesale supply of pharmaceutical products to pharmacies, hospitals or institutions. It authorizes the bulk sale of drugs to licensed entities—not directly to end consumers. This license is also issued by the State Drug Control Authority. You must appoint a qualified person, who can be either a registered pharmacist, a science graduate with one year of experience or someone with four years of pharmaceutical sales experience. The premises should be at least 15 square meters and must meet hygiene and storage standards, including cold storage if dealing in sensitive drugs. The application process includes submitting business incorporation documents, qualifications/experience of the competent person, premises proof and payment of license fees. Upon approval, you receive Form 20B and Form 21B, which cover general and restricted drugs for wholesale. The license is valid for 5 years and must be renewed thereafter. Wholesalers are not permitted to sell directly to the public—only to other licensed entities.
- Additional Licenses or Endorsements: Depending on the range of products and services you plan to offer, there could be other specific licenses needed:
- Schedule X Drugs: Selling habit-forming or narcotic drugs under Schedule X requires a separate license via Form 19C. Most pharmacies avoid it due to strict compliance.
- Import License: Importing medicines or representing foreign manufacturers needs a CDSCO Import License, separate from standard pharmacy licensing.
- Medical Devices/Cosmetics: Most basic devices and cosmetics can be sold under a pharmacy license, but regulated devices may require separate compliance under medical device rules.
For most retail and wholesale pharmacy businesses dealing in standard allopathic medicines, the primary licenses will be the retail/wholesale drug license as described. Always keep copies of all licenses at the premises and adhere to any conditions mentioned (such as maintaining purchase/sales records, pharmacist presence during dispensing hours, etc.).
Note: Drug licenses in India are location-specific and entity-specific. This means if you open another branch or move premises, you must obtain a new license for the new location. Also, if you expand to another state, you will need to apply afresh in that state (there is a concept of a “multi-state license” or multi-drug license for chains, but essentially it entails holding individual licenses in each state of operation under the same name). Always update the authorities if any detail changes (like a change in pharmacist or change of firm name), to keep your license valid.
WHY APPROVALS AND LICENSES ARE NEEDED
You might wonder why there is such an elaborate process of approvals and licensing to start a pharma business. The reason is public health and safety. Medicines are not ordinary consumer goods – selling them improperly can have serious consequences. Thus, the government strictly regulates this sector to ensure that only qualified businesses and professionals handle drugs. Here’s why these approvals and licenses are essential:
- Protecting Health and Safety: Drug laws ensure only qualified pharmacists dispense medicines, preventing misuse of antibiotics or narcotics. Licensing enforces prescription protocols and expert oversight, safeguarding lives by ensuring dangerous drugs aren’t sold freely or without medical guidance.
- Ensuring Quality and Proper Storage: Licenses mandate clean premises, refrigeration and proper infrastructure, protecting medicine quality. Inspections verify storage standards, ensuring customers receive authentic, effective and non-expired drugs. Licensed pharmacies thus provide safer, regulated medicine handling and reduce health risks.
- Accountability and Traceability: Licensed pharmacies maintain purchase and sales records, enabling drug traceability and recall if needed. Operating without a license invites criminal action. These rules deter counterfeit sales, ensuring public safety through documented supply chains and regulated medicine distribution.
- Preventing Misuse of Controlled Substances: Special licenses for narcotics or Schedule X drugs prevent over-the-counter abuse. Only select, vetted pharmacies can sell them under strict conditions, curbing addiction, illegal sales and protecting society from harmful misuse of habit-forming medications.
- Legal Compliance and Business Continuity: Licensing ensures regulatory peace of mind, uninterrupted operations and access to suppliers. Unauthorized sales risk shutdowns, raids or legal penalties. A licensed setup builds trust with consumers and pharmaceutical distributors, ensuring smooth business functioning and credibility.
In summary, these approvals and licenses are needed to uphold standards in the pharma industry. They protect consumers, ensure only trained persons dispense medicine and keep substandard or dangerous drugs in check. While the process may seem extensive, it ultimately contributes to the reputation of your business as a safe and reliable source of medications. Compliance with the law is not just a duty but a cornerstone of running a successful healthcare enterprise.
HOW LAWFINITY CAN HELP YOU ESTABLISH YOUR PHARMA BUSINESS
Opening a pharmacy in India is a matter of dealing with a very complex format of working which is being regulated by several central and state government departments as well. We, at Lawfinity, specialize in assisting entrepreneurs, distributors and pharmacy owners, setting-up and running fully compliant pharma business, be it retail or wholesale. With expertise in Drugs and Cosmetics Act, State Drug Control Regulations and relevant tax & labour laws, we are capable of providing you end to end consultancy, filing and advisory services specifically designed for pharmaceutical industry.
Here’s how we assist you at every step:
- Business Incorporation and Structuring : We help you choose and register the right legal entity—such as a Private Limited Company or LLP—under the Ministry of Corporate Affairs (MCA). Our experts handle all necessary filings including PAN, TAN, GST and MSME registration, ensuring you begin operations with a legally recognized structure that’s acceptable to licensing authorities.
- Drug License Application for Retail and Wholesale : We prepare and submit your applications for Form 20, 21 (retail) and Form 20B, 21B (wholesale) licenses, ensuring all documents, affidavits and declarations meet the Drug Controller’s requirements. We also assist in site inspection readiness and compliance.
- Pharmacist and Competent Person Onboarding : Whether you are a non-pharmacist owner or expanding into wholesale, we assist in the recruitment and verification of qualified pharmacists or competent persons. We prepare appointment letters, qualification records and submit them with your license file.
- Premises Setup and Compliance Guidance : Our team guides you on selecting a compliant location that meets Drug Department norms—including minimum area, air-conditioning, refrigeration and storage layout. We also assist in preparing rent agreements, property ownership proofs and blueprint approvals.
- GST and FSSAI Registration : If you are dealing in health supplements, nutraceuticals or medical consumables, we help you obtain the necessary FSSAI License. We also manage your GST application and advisory, essential for tax invoicing and supply chain integration.
- State Drug Department Coordination : We act as your single point of contact with State Licensing Authorities, handling documentation submission, fee payments, site inspection scheduling and addressing any objections or queries raised by officials until final approval is secured.
- Legal Drafting and Documentation : Our legal experts draft all supporting documents, including lease deeds, sale agreements, appointment letters, vendor contracts, non-disclosure agreements and purchase orders—ensuring your paperwork is accurate, professional and enforceable.
Whether you are launching your first medical store, expanding into multi-location distribution or transitioning into franchise-based pharma trade, Lawfinity India ensures you are legally prepared, fully compliant and market-ready.
We take care of the heavy lifting—so you can focus on procurement, branding, sales and growth without the fear of regulatory hurdles.
Looking to start your pharma business the right way?
Visit www.lawfinity.in or reach out to our expert team today for a free consultation.
Let Lawfinity be your trusted legal partner in building a compliant and successful pharmaceutical venture.
Indian Pharmaceutical Industry – A Decade of Resilience and the Road Ahead
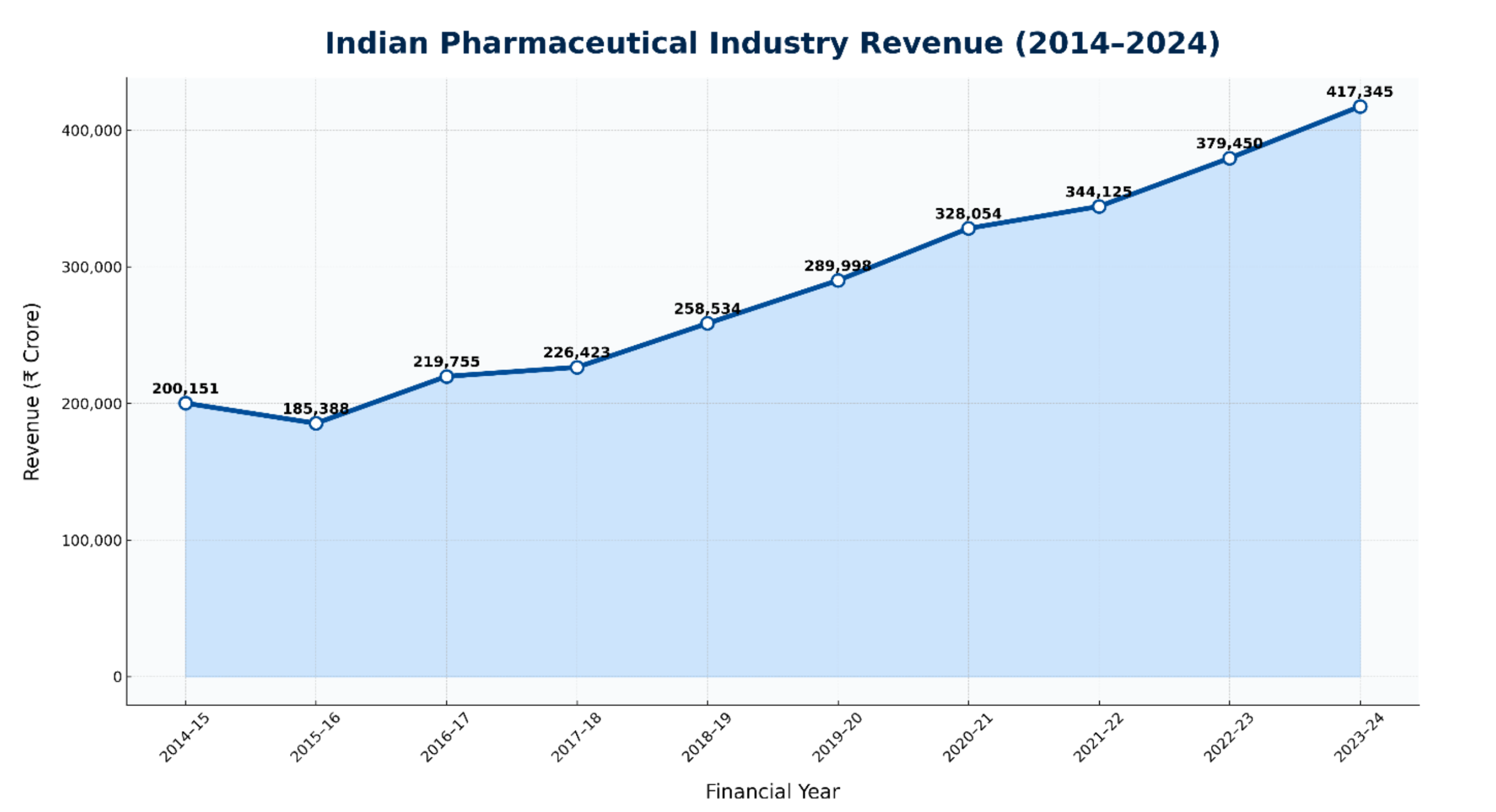
Source: Ministry of Chemicals & Fertilizers (Dept. of Pharmaceuticals) Annual Reports
- Strong Foundation in Generics
India started with ₹2,00,151 crore revenue in 2014–15, led by generics and global reach.
- Resilience Amid Regulatory Pressure
Despite a dip in 2015–16 to ₹1,85,388 crore, Indian pharma bounced back through R&D and compliance improvements.
- Pandemic-driven Growth Surge
Post-2020, domestic and global demand surged; the industry peaked at ₹4,17,345 crore in 2023–24.
AUTHOR’S OPINION: PAST, PRESENT & FUTURE OF PHARMA INDUSTRYThe Past: A Foundation Built on Generics and Global Reach
A decade ago, India’s pharmaceutical industry was predominantly known for its generic drug exports. With low-cost manufacturing and a skilled scientific workforce, Indian companies became global suppliers, especially to regulated markets like the US, Europe and Africa. However, between 2014–16, there were signs of turbulence. Increased scrutiny from international regulators (like the USFDA), price erosion and patent disputes slightly dented the industry’s momentum — as seen in the 2015–16 revenue dip.
Still, the core foundation remained strong. Indian firms quickly responded by strengthening compliance, exploring new markets and investing in better R&D facilities. This adaptability laid the groundwork for future stability.The Present: Acceleration Post-Pandemic
The turning point came around 2020, when the global pandemic placed India in a pivotal role — as a vaccine manufacturer, supplier of essential medicines and API producer. The government’s PLI scheme, push for Atmanirbhar Bharat and reforms in healthcare infrastructure directly contributed to consistent revenue growth year after year.
With the launch of digital health missions, rapid urbanization and increasing insurance penetration, domestic demand has surged. Indian pharma is now no longer just a volume player; it’s moving into value-based markets such as:
- Biosimilars
- Oncology
- Vaccines
- Wellness & nutraceuticals
Revenue rose from ₹2.9 lakh crore in 2019–20 to over ₹4.1 lakh crore in 2023–24 — a clear sign of sectoral maturity.The Future: From the World’s Pharmacy to a Global Innovation Hub
Looking forward, India has immense opportunities — but also new responsibilities.
Growth Drivers:
- Global demand for affordable and high-quality drugs.
- Shift towards innovation in drug delivery systems and biotech research.
- Increasing investments in AI, digital therapeutics and personalized medicine.
- Expansion into Latin America, Southeast Asia and Eastern Europe.
Challenges:
- Regulatory reforms and faster drug approvals.
- Tackling pricing control issues.
- Enhancing skill development and academic–industry collaboration.
If these aspects are addressed, India can transition from being a generic leader to a holistic global healthcare provider.CONCLUSION AND EXPERT OPINION: A JOURNEY OF RESILIENCE, READY FOR REINVENTION
In my opinion, the Indian pharmaceutical industry stands as a beacon of resilience — having weathered regulatory storms, global scrutiny and pandemic disruption, yet emerging stronger each time. The data proves not just growth in numbers but evolution in capability.
We are at the cusp of a golden opportunity. With the right ecosystem — policy support, infrastructure, talent and investment — India can lead the global pharma space not just as a supplier, but as an innovator, collaborator and trailblazer.
The last decade proved India can lead in medicine supply. The next decade will show whether we can lead in medical innovation.
Frequently Asked Questions
Because every great business starts with the right answers.
A retail pharmacy must have a minimum 10 m² area; wholesale or combined operations require 15 m². Authorities verify premises through a layout plan before issuing a license, ensuring compliance with space and storage norms.
Yes, a registered pharmacist is mandatory for retail pharmacies, though ownership can be by a non-pharmacist. The pharmacist must be registered with the State Pharmacy Council and present during business hours to supervise sales and guide customers.
Retail licenses permit selling medicines to the public via prescriptions; wholesale licenses allow sale in bulk to licensed entities only. Retail needs a pharmacist and 10 m² area; wholesale requires a competent person and 15 m² premises.
State Drugs Control Authority (or FDA) issues retail and wholesale drug licenses. Applications go to the district Drug Licensing Authority. For manufacturing/import, CDSCO is involved. Each state operates its own system, often through online portals.
It usually takes 30–60 days after application and inspection to receive a license. A drug license is valid for 5 years and must be renewed before expiry to continue legal pharmacy operations without disruption.
Starting a retail pharmacy typically requires ₹3–8 lakhs, covering rent, furniture, refrigeration, initial stock and licensing. Larger or wholesale setups need more. Have a financial buffer for at least 6–12 months of initial operations.
Yes, pharmacies are generally profitable due to consistent demand for medicines. Profitability depends on location, product range, sourcing discounts and regulatory compliance. Smart inventory and good service increase customer retention and long-term returns.
Yes, each pharmacy location requires its own drug license and pharmacist. Operating in multiple states means separate licenses per state. Compliance is site-specific, even for chains or branches under one corporate entity.
GST registration is mandatory if annual turnover exceeds ₹40 lakhs (₹20 lakhs in special states) or for inter-state sales. Voluntary registration allows input credit and smoother transactions with suppliers. Most medicines attract 5% GST.